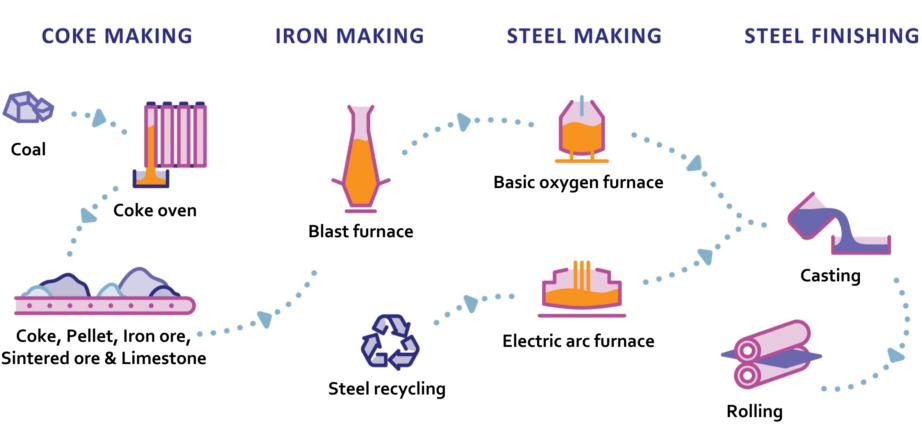
Coke
Coking coal is converted to coke by driving off impurities to leave almost pure carbon.
The coking coal is crushed and washed. It is then ‘purified or ‘carbonised in a series of coke ovens, known as batteries, where the coking coal is heated to 1000-1100ºC in the absence of oxygen for 12-36 hours. During this process, by-products are removed, and coke is produced.
Iron
During the iron-making process, a blast furnace is fed with the iron ore, coke and small quantities of fluxes (minerals, such as limestone, which are used to collect impurities). Air which is heated to about 1200°C is blown into the furnace through nozzles in the lower section. The air causes the coke to burn, producing carbon monoxide which reacts with the iron ore, as well as heat to melt the iron. Finally, the tap hole at the bottom of the furnace is opened and molten iron and slag (impurities) are drained off.
Steel production
Steel is produced via two main routes: the blast furnace-basic oxygen furnace (BF-BOF) route and electric arc furnace (EAF) route. The BF-BOF route is used for 71% of steel produced, whilst the EAF route accounts for 29% of steel produced. The key difference between these routes is the material used to produce the steel and the plant configuration.